Copyright © Karl Dahlke, 2022
Hold a piece of lego in your hand. You can't cut it in half, nor change its shape, it is what it is, but you can observe its structure. It isn't a featureless ball of matter. It has dots above and indentations below, to facilitate connections with other lego blocks, and it is mostly empty space. Atoms are the same way. Democritus knew that; if atoms didn't have hooks, then how could they connect to each other? If atoms were featureless spheres then all matter would be gases, like the nobel gases, and life could not exist. His intuition was spot on!
Before we talk about the components of an atom, we need to define electric charge. Magnets can help, but in some ways they confuse. Find 2 free magnets in your home. (A magnet embedded in a picture, to hang on the refrigerator, is not very helpful.) A free magnet has a north and a south pole. Bring two magnets together and see what happens. Opposite poles attract, thus the south pole of one magnet pulls on the north pole of the other, until they slap together. Hold both magnets with their north poles toward each other and watch how they push each other apart. Place both magnets on a table with their north poles pointing toward each other, then release one of the magnets. With its north pole pushed away, it spins around, bringing the south pole to bear. With north facing south, the magnets slap together. Repeat this a few times until you are comfortable with magnets and their polarity.
The center of an atom contains protons, and each proton has a positive electric charge. You might think of this as the north pole of a magnet, whence positive charges repel each other. Electrons whirl around the outside of an atom, and electrons have a negative charge. You might think of each electron as the south pole of a magnet; negative charges repel each other. And of course, positive and negative charges attract, like the north and south poles of two magnets. But the magnet analogy is far from perfect. A proton doesn't have a positive charge and a negative charge, like two ends of a magnet. It is a single sphere of positive charge. In the same way, an electron is a single sphere of negative charge.
Along with protons and electrons, atoms contain neutrons, which have no charge. A neutron does not attract or repel electrons or protons, or other neutrons. The word neutron comes from neutral, having no charge.
The lighter atoms have roughly the same number of neutrons and protons, give or take a few. Heavier atoms have a few more neutrons, e.g. uranium has 92 protons and 143 neutrons.
Protons, neutrons, and electrons are called subatomic particles because they are smaller than an atom. Sub is Latin for below, as in submarine (below the water), substandard (not good enough), subterranean (below the ground), subconscious (below the level of consciousness), etc.
Every atom has just as many protons as electrons. If it didn't, it would be out of balance. If an oxygen atom has 8 protons and 7 electrons, it has a net positive charge. The extra proton means the atom has a charge of +1. This is called an ion, a positive ion. If an electron is passing by, it is attracted to this ion, and is sucked in like a magnet. With 8 electrons, the oxygen atom is happy once again. Now e get the joke, “ION television, positively entertaining.” Actually there's a funnier and older joke, perhaps the best pun ever. Two hydrogen atoms were walking down the street. One says to the other, “I think I lost my electron.” The second asks, “Are you sure?” The first says, “Yes, I'm positive.”
Conversely, suppose an oxygen atom has 8 protons and 9 electrons. The extra electron gives it a net charge of -1, thus it is a negative ion. It can easily lose its electron, perhaps to a nearby positive ion, whereupon it becomes a balanced oxygen atom once again. Ions are important in chemistry, but for now, let's assume atoms are balanced, with the same number of protons and electrons, and no net charge.
Neutrons have no effect on the charge of an atom, thus some variations are allowed. A carbon atom always has 6 protons and 6 electrons, but it could have 6, 7, or 8 neutrons. These are called isotopes, different forms of the same element. You'd hardly notice however. Neutrons live at the center of the atom, and electrons live at the outer edge, where chemistry takes place. Carbon is carbon, and it builds hydrocarbons and carbon dioxide and all the other compounds you know, no matter how many neutrons are at the core.
An atom is so incredibly tiny, you'd think it was a solid ball of matter, but it is mostly empty space, with protons, neutrons, and electrons forming its framework. Recall that the width of an atom is the with of your finger divided by 100 million. Expand a carbon atom to the size of a football field. Each proton and neutron is now the size of a grain of sand, and they are huddled together in a small ball on the 50 yard line, 12 little grains of sand that anchor the entire atom. This ball is called the nucleus. You thought atoms were small - protons and neutrons are even smaller!
Continuing the football field analogy, two electrons whip around the nucleus at a distance of 25 yards, and the other four electrons whirl around at a distance of 50 yards, just inside the goal posts. How big are the electrons? Still too small to be seen. Each electron could be 1000 times smaller than a grain of sand. We really don't know, or perhaps can't define, the size of an electron. It may be pointlike, having no size as we understand it, or it may smear out across the atom in a quantum mechanical wave. In fact the latter is probably the case. Each electron is a tenuous cloud, spreadout around the outside of the football field, and there's no way to pin down the electron's location, or determine its size. But since it has a charge, we know how many there are. Assuming the carbon atom is properly balanced, there are six electrons, each forming its own cloud, thus balancing the six protons in the nucleus.
The proton has 1836 times the mass of the electron, hence almost all of the mass of an atom is in the nucleus. Our enlarged atom covers a football field, yet all the mass is in those 12 tiny grains of sand at the center.
The atomic number is the number of protons, and the atomic weight is the number of protons and neutrons, since they comprise the "weight" of the atom. Electrons have very little mass, and contribute almost nothing to the weight of the atom. However, electrons determine the chemistry, Thus each element is indicated by its atomic number, the number of protons and the number of electrons. Isotopes of the same element have different numbers of neutrons, variations on a theme, but virtually the same chemistry. In an earlier chapter I presented a list of elements in order, though the order was not clear at that time. Now we understand - the elements are sorted by atomic number, by the number of protons in the nucleus. Hydrogen has just one proton, helium has 2, carbon has 6, oxygen has 8, and so on. Let's look at the list again. This time I'll include the atomic weight, which is the average number of protons and neutrons. This is just an average, some atoms have fewer neutrons, some have more. It is a fraction, as you would expect from an average, but of course each atom has a whole number of neutrons. The vast majority of carbon atoms have 6 neutrons, some have 7, and some have 8, thus an average of 12.0107.
| 1 | Hydrogen | H | 1.0079 |
| 2 | Helium | He | 4.0026 |
| 3 | Lithium | Li | 6.941 |
| 4 | Beryllium | Be | 9.0122 |
| 5 | Boron | B | 10.811 |
| 6 | Carbon | C | 12.0107 |
| 7 | Nitrogen | N | 14.0067 |
| 8 | Oxygen | O | 15.9994 |
| 9 | Fluorine | F | 18.9984 |
| 10 | Neon | Ne | 20.1797 |
| 11 | Sodium | Na | 22.9897 |
| 12 | Magnesium | Mg | 24.305 |
| 13 | Aluminum | Al | 26.9815 |
| 14 | Silicon | Si | 28.0855 |
| 15 | Phosphorus | P | 30.9738 |
| 16 | Sulfur | S | 32.065 |
| 17 | Chlorine | Cl | 35.453 |
| 18 | Argon | Ar | 39.948 |
| 19 | Potassium | K | 39.0983 |
| 20 | Calcium | Ca | 40.078 |
| 21 | Scandium | Sc | 44.9559 |
| 22 | Titanium | Ti | 47.867 |
| 23 | Vanadium | V | 50.9415 |
| 24 | Chromium | Cr | 51.9961 |
| 25 | Manganese | Mn | 54.938 |
| 26 | Iron | Fe | 55.845 |
| 27 | Cobalt | Co | 58.9332 |
| 28 | Nickel | Ni | 58.6934 |
| 29 | Copper | Cu | 63.546 |
| 30 | Zinc | Zn | 65.39 |
| 31 | Gallium | Ga | 69.723 |
| 32 | Germanium | Ge | 72.64 |
| 33 | Arsenic | As | 74.9216 |
| 34 | Selenium | Se | 78.96 |
| 35 | Bromine | Br | 79.904 |
| 36 | Krypton | Kr | 83.8 |
| 37 | Rubidium | Rb | 85.4678 |
| 38 | Strontium | Sr | 87.62 |
| 39 | Yttrium | Y | 88.9059 |
| 40 | Zirconium | Zr | 91.224 |
| 41 | Niobium | Nb | 92.9064 |
| 42 | Molybdenum | Mo | 95.94 |
| 43 | Technetium | Tc | 98 |
| 44 | Ruthenium | Ru | 101.07 |
| 45 | Rhodium | Rh | 102.9055 |
| 46 | Palladium | Pd | 106.42 |
| 47 | Silver | Ag | 107.8682 |
| 48 | Cadmium | Cd | 112.411 |
| 49 | Indium | In | 114.818 |
| 50 | Tin | Sn | 118.71 |
| 51 | Antimony | Sb | 121.76 |
| 52 | Tellurium | Te | 127.6 |
| 53 | Iodine | I | 126.9045 |
| 54 | Xenon | Xe | 131.293 |
| 55 | Cesium | Cs | 132.9055 |
| 56 | Barium | Ba | 137.327 |
| 57 | Lanthanum | La | 138.9055 |
| 58 | Cerium | Ce | 140.116 |
| 59 | Praseodymium | Pr | 140.9077 |
| 60 | Neodymium | Nd | 144.24 |
| 61 | Promethium | Pm | 145 |
| 62 | Samarium | Sm | 150.36 |
| 63 | Europium | Eu | 151.964 |
| 64 | Gadolinium | Gd | 157.25 |
| 65 | Terbium | Tb | 158.9253 |
| 66 | Dysprosium | Dy | 162.5 |
| 67 | Holmium | Ho | 164.9303 |
| 68 | Erbium | Er | 167.259 |
| 69 | Thulium | Tm | 168.9342 |
| 70 | Ytterbium | Yb | 173.04 |
| 71 | Lutetium | Lu | 174.967 |
| 72 | Hafnium | Hf | 178.49 |
| 73 | Tantalum | Ta | 180.9479 |
| 74 | Tungsten | W | 183.84 |
| 75 | Rhenium | Re | 186.207 |
| 76 | Osmium | Os | 190.23 |
| 77 | Iridium | Ir | 192.217 |
| 78 | Platinum | Pt | 195.078 |
| 79 | Gold | Au | 196.9665 |
| 80 | Mercury | Hg | 200.59 |
| 81 | Thallium | Tl | 204.3833 |
| 82 | Lead | Pb | 207.2 |
| 83 | Bismuth | Bi | 208.9804 |
| 84 | Polonium | Po | 209 |
| 85 | Astatine | At | 210 |
| 86 | Radon | Rn | 222 |
| 87 | Francium | Fr | 223 |
| 88 | Radium | Ra | 226 |
| 89 | Actinium | Ac | 227 |
| 90 | Thorium | Th | 232.0381 |
| 91 | Protactinium | Pa | 231.0359 |
| 92 | Uranium | U | 238.0289 |
| 93 | Neptunium | Np | 237 |
| 94 | Plutonium | Pu | 244 |
Now we can gain an intuitive understanding of why atoms are the same size. Bring in more protons and more electrons and watch what happens. Electrons repel each other, so they can't clump together. They whirl around the nucleus in wider and wider orbits. However, there are ever more protons, thus a higher positive electric charge at the center of the atom. This charge pulls the electrons closer to the nucleus, in tighter orbits. These effects cancel each other out. The outermost orbit determines the size of the atom, and therefore, all the atoms are roughly the same size.
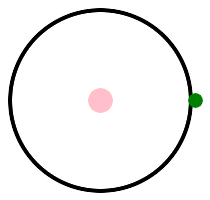
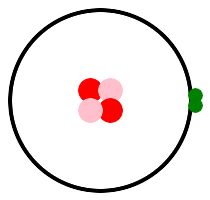
Let's look at some pictures. Hydrogen is on the left, with one proton, and helium is on the right, with two protons. This is helium 4, 2 protons and 2 neutrons. There is another isotope, helium 3, with 2 protons and 1 neutron. Protons are pink, neutrons are red, and electrons are green. p stands for pink, or proton, or positive charge.
| Hydrogen H | Helium He | |
|---|---|---|
 |
 |
If you've been following along, you have some questions / concerns. Why are the two electrons in helium right next to each other? Don't they repel each other? Electrons have a property that we call spin, though the analogy to spinning is not perfect. If one electron spins one way and another electron spins the other way, they can attract, and will travel together as a pair. Most electrons group together in pairs. Three electrons will never clump together. We'll see this in the oxygen atom below.
What about the protons in the nucleus? There could be dozens of protons practically touching each other; why doesn't the nucleus fly apart? There is a short-range force, stronger than electric charge, that holds the nucleus together. It is called the strong nuclear force, simply because we don't know what else to call it. It applies to neutrons and protons alike, and it holds the nucleus together in a tight ball. It only extends for a short distance however, about an inch on the football field, thus nuclei can only grow so big, before the outermost protons fly away due to electric charge.
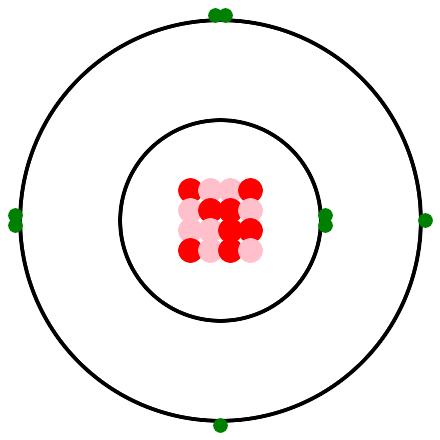
An unpaired electron provides the hook that Democritus envisioned. Hydrogen has one unpaired electron, thus one bond. Helium has no unpaired electrons, thus no bonds. Helium is a nobel gas. We saw in the introduction to atoms, that oxygen has 2 bonds. Thus oxygen joins with two hydrogens to make a water molecule. Here is a depiction of an oxygen atom with 2 unpaired electrons, thus 2 bonds. In the real world, the nucleus isn't a neat 4 by 4 array, in fact it doesn't lie flat on the page, It is an amorphous ball of 8 protons and 8 neutrons. Also, the electrons and electron pairs don't lie flat on the page, they whirl around in spheres, which are called electron shells.
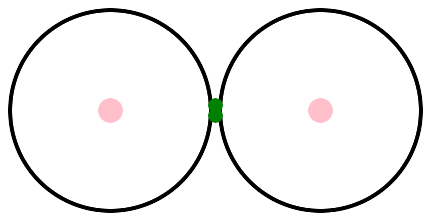
Let's hook two atoms together to make a molecule. The simplest molecule is H2. After the connection, you can't tell which electron came from which atom. They are simply a pair of electrons, shared between two atoms.
Element number 29 is copper, and it conducts electricity well, thus wires are often made of copper. The atoms in a metal don't form self-contained molecules, rather, they form a bulk solid. Each is connected to its neighbors by electron bonds. If an electric field is applied, an electron can jump from one copper atom to the next. This leaves a positive copper ion behind, but within a period of time that is too small to measure, an electron jumps from the previous copper atom to this one, and it is balanced once again. Sure enough, this electron moves along, and another one takes its place. Electrons move around the circuit like a conveyer belt, and perhaps they power a lamp or run an appliance along the way. Electrons are so named because they convey electricity - only later did we discover their role in atoms and in chemistry. There's much more to say about electricity and electrical circuits, but that will have to wait for another day.
Instead of 12 grains of sand at the center of the football field, what if the entire field was covered in sand, 50 yards high? How does all that sand compare to 12 little grains? We can't pack that much nuclear material together, but if we could, a cubic centimeter, about the size of a marble, would weigh 100 million tons. It would sink to the center of the earth, drilling through rock and iron like a knife through butter. Expand ordinary matter, like your kitchen table, so that each atom is a football field, and you find field after field after field, each with a few grains of sand at the center; but expand nuclear material and it's sand everywhere, packed tightly together. We can't create this compact material in the lab, but a star can. After a star's fuel is exhausted, and it shines no more, it can, under certain conditions, collapse down to a neutron star. Its unimaginable weight crushes the atoms together, and pushes the electrons down onto the nucleus. Protons and electrons combine, and produce neutrons, with no charge. The result is a giant ball of neutrons, 20 kilometers across, about the size of a city. There is no empty space here. Gravity is 200 billion ɡ's at the surface, 200 billion times the gravity of earth. A housefly would weigh 2 thousand tons. If an object falls from a height of one meter, it hits the ground at a speed of 1400 kilometers per second. Neutron stars are endlessly fascinating; you can read about them here.
Obviously we're never going to send a human, or even a probe, anywhere near a neutron star, so let's perform a thought experiment. suppose we get lucky, and a tiny chunk of neutron star material, with the mass of Mount Everest, passes through our solar system. You fly up to investigate as it sails by the earth. It is a sphere a little bigger than a baseball, 6.2 centimeters, or 2.4 inches, in radius. Could you hold it at arms length, or would it suck you in? Bad news; at a distance of one meter, the baseball pulls you towards it with a force of 660 ɡ's. Let's reverse engineer it. Pick a smaller mountain, so that you feel 1 ɡ of force, like you do here on earth. The neutron sphere is now 1.4 cm in diameter, the size of a large marble. You can see it floating there in front of you. A metal support, one meter long, extends out from this sphere and holds a platform that you are lying on, so you don't get sucked in. It's a comfortable 1 ɡ, like you are lying in bed on earth. If you stand up and jump up and down on the platform, the marble, due to its tremendous mass, doesn't move. It gives the appearance of being anchored in space. You can't disturb its trajectory in any way. Now, what if you are curious - what if you reach out and touch the marble? You wonder what neutron star material feels like. Is it hot or cold, rough or smooth? Nobody has ever touched neutronium before. Unfortunately, gravity at the surface of the sphere is 20,000 ɡ's. Your hand is crushed onto the surface of the marble, shattering bones in the process. A layer of pulverized, compressed flesh wraps around the surface of the marble. It is impossible to pull your arm away; it has an effective weight of 20,000 pounds. Blood also has 20,000 times its normal weight, and it bursts through the veins and arteries. it is sucked out of your arm like a vacuum cleaner, and you would quickly bleed to death. So don't reach out and touch the marble! Observe it from your platform, never getting closer than one meter, or drop something onto it if you want to see some fireworks.