Copyright © Karl Dahlke, 2022
Mercury was an essential component of the early thermometers and barometers, which measured temperature and atmospheric pressure in the 17th century. The previous chapter described heat and pressure at a molecular level, and the relationship between temperature, pressure, and volume, but science is nothing without observations, so let's see how mercury made that possible.
Hydrargyrum was an early name for mercury, thus its symbol is Hg. Yeah, it's one of those symbols you just have to memorize.
Almost all elements are solid at room temperature and pressure. 11 elements are gases, and only 2 are liquid, mercury and bromine, although 3 others, caesium, gallium, and rubidium, melt just above room temperature. Mercury melts at -37.8 °F, -38.8 °C, and boils at 674 °F, 357 °C, thus it remains liquid across a wide range of temperatures. In addition, mercury is a metal, and liquid metals are extremely rare. Until recently, no other liquid metals were known. Mercury is silvery-white in color, and is also called quicksilver, i.e. a silver metal that moves about.
A modern application of mercury is the mercury switch. If you've ever had a space heater, you may have noticed a safety feature; its shuts off automatically if it is knocked on its side. This prevents it from pouring heat into the floor and starting a fire. The switch that makes this possible has no moving parts; it is simplicity itself. A small amount of mercury sits at the bottom of a closed tube with a metal contact on either side. Electricity passes through the mercury and runs the appliance. If the unit is tipped to one side or the other, or upside down, the mercury flows into its new position and does not touch both contacts any more. The circuit is broken and the appliance stops immediately. You can install two such switches if you like, one for a left right tilt and one for a front back tilt. In this illustration, the mercury is dyed red, just as it is in thermometers. Remember, its natural color is silver.

We know, by the previous chapter, precisely how a gas expands when heated; twice as hot expands to twice the volume, etc. Solids and liquids expand as well, but not nearly as much. Take an iron bar one meter long (or one yard long) and toss it into a fire until it becomes red hot. Its length increases by about 1%, one centimeter, or half an inch. volume increases by the cube of this ratio, or 3%. This is much less than a heated gas, which would expand by a factor of 4 under Charles' law. Although the thermal expansion of a metal is far less, it is approximately linear, which is useful in many applications, including the thermometer.
The design of a mercury thermometer is simple, yet elegant. Place a reservoir of mercury at the bottom of a closed, glass tube. Add red dye to the mercury so it can be seen clearly through the glass. At the lowest temperature that the thermometer can measure, the mercury precisely fills the chamber at the bottom. Heat the mercury by one degree and it expands a tiny amount, a few parts per million, far too little to see. However, mercury is a liquid, and it squeezes out the top of the chamber and climbs up a very narrow tube. The volume changes by a tiny percent, but that change can be seen climbing the tube. Each degree is another change in volume, assuming linear expansion, and that is another step up as the red liquid climbs the tube. This continues up to the hottest temperature the thermometer can record. It is important to maintain a vacuum in the thermometer, since any air in the tube would push back against the rising mercury and lower the reading, or it might cause the thermometer to explode.
Mercury is 13.5 times as dense as water, and is the densest liquid known. Suppose you stepped into a bathtub full of mercury. (Mercury is safe to touch, assuming you wash it off thoroughly, but don't ingest it, or breathe in mercury vapors, as it is poisonous.) The silvery liquid is 13.5 times as dense as you are, thus you can almost float atop the mercury, with just 7.4% of your body immersed, perhaps your buttocks and lower legs. High density is important in the next application.
Numbers in this chapter are approximate for readability; I'm not going to quibble about a few percent. They vary anyways, depending on where you live.
It's fun to drink through a straw, but have you tried it in a swimming pool? You don't actually want to drink the chlorinated water, but there is an opportunity for science here. Find a long straw, perhaps one of those floatation tubes, or any other tube that you don't mind putting your mouth on, and suck the water up the tube as though you were drinking through a very long straw. It doesn't have to be very long however. No need to stand on the diving board. You can only suck the water 3 feet (one meter) up the tube, 3 feet above the level of the pool. At that point your lungs are straining, because the weight of the water is pulling down as hard as your diaphragm can pull up. By analogy, your right arm might be able to lift 50 pounds against the pull of the earth, and no more.
It doesn't matter if the straw is as thin as a pencil, or as wide as your mouth, or (theoretically) as wide as a stadium; you can only pull water 3 feet up the tube. If the straw is wide, it might take several breaths to coax the water up the tube. The first breath is easy, as the water rises a few inches. The next breath is not too difficult, but after a while it's hard going, until the weight of the water per square inch is the same as the pull of your diaphragm on the air in the tube, per square inch. If the straw is narrow, like a drinking straw, the water jumps up 3 feet in half a breath, but the limit is the same. Try this experiment with different size tubes the next time you're in a pool. You could probably do it with a bucket of water and a vacuum cleaner hose at home.
So how strong are your lungs anyways? Can we put a number on it?
Use a straw that is 4 feet tall (you can only suck water up 3 feet anyways), and a little more than an inch wide. The cross sectional area of your straw is one square inch. When the water has climbed 3 feet up the straw, you are holding up, with your breath, the weight of a column of water 3 feet high and 1 square inch in cross section. This is 36 cubic inches of water, weighing about 1.3 pounds. You can suck air in with a force of 1.3 pounds per square inch, or 1.3 psi.
You have a friend name Bill who always tries to best you at every turn. You know the type. He comes over with a 40 foot straw and says, “Watch this!”, as he stands on the roof of your two story house. He attaches a vacuum pump to the top of the tube and turns it on. All the air is sucked out of the tube, which is, fortunately, made of glass so it does not collapse under pressure. (You've probably tried to drink a thick milk shake through a paper or plastic straw and had it flatten on you.) His straw is just as wide as yours, 1 square inch in cross section. The water climbs up the tube, but stops at 33 feed, or 10 meters. It can climb no higher, even though all the air has been sucked out of the tube. This column of water, 33 feet high and 1 square inch across, weighs 14 pounds. Since it is holding steady, everything must be in balance. The column of air over every other square inch of your swimming pool must also weigh 14 pounds. If it weighed more, it would push the water up higher in the tube. Imagine a column of air, 1 square inch across, like the circle made by your finger and thumb, but the column goes all the way up to the sky. Miles and miles of air, past the clouds, all the way up to space. Air is lighter than water of course, but this column is miles high. It weighs 14 pounds, just like the column of water in Bill's tube. Bill has discovered a handy way to measure ambient air pressure.
Well not so handy really - he has to use a modern vacuum pump, and stand precariously on your roof. Soon Cathy comes along with a better idea. She has a long glass tube, like Bill's, but it is sealed at one end. She lays this tube down in your pool until it is completely under water. (You've got a really long pool.) She tips the open end up just a bit, but still holds it below the surface, so all the air runs out. After the bubbles stop, there is nothing but water in the tube. Now she points the open end down, and lifts the closed end up out of the water. Soon the tube is standing straight up with its open end still in the water and its closed end at the top. The tube rises 40 feet above the surface of the pool, but the water is only 33 feet high in the tube. Just like bill's tube, the water can only rise 33 feet above the surface of the pool. This balances the air pushing down on the rest of the pool. The last 7 feet of tube have no air and no water - a pure vacuum. “See,” she smiles, “I didn't need a vacuum pump to suck all the air out, and I don't have to stand on your roof. I can do it all from your swimming pool.” (Cathy is pretty strong, and can swing a 40 foot glass tube about without breaking it.) She has made the same empirical measurement as Bill. Atmospheric pressure at your elevation is 33 feet of water, or 14 psi.
Gasparo Berti performed this very experiment in 1640, though he didn't understand why the water stopped at 33 feet. He thought air was weightless, as did everyone in his day, even Galileo.
If you happen to live on Venus, which has a much thicker atmosphere, you would need a tube over half a mile long. Pressure on Venus is 90 earth atmospheres. But the water in your swimming pool would boil away anyways, so no matter.
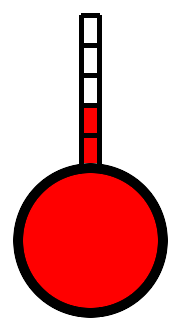
At this point, Evan comes along with a tabletop apparatus. His tube is only 3 feet long. Again it is made of glass and sealed at one end. He lays it in a red liquid and tips the open end up until all the air runs out. He then tips the open end down and lifts the closed end up out of the liquid. The tube stands 3 feet high, but the liquid, which is much easier to see than water, rises just 30 inches up the tube. The last 6 inches are empty. “This is called a barometer,” he explains, “and it uses mercury, which is heavier than water. It's the heaviest liquid known. So this column of liquid, 30 inches high, weighs the same as 33 feet of water, or miles and miles of air.”
Evangelista Torricelli performed this experiment in 1644, and surmised, correctly, that air had weight, and the atmosphere exerts pressure on us all, pressure that exactly balances the 30 inches of mercury in his glass tube. (Mercury is not actually red, but a dye is added to make it easier to see.)
Other 17th century scientists soon learned that the pressure is less at the top of a mountain, since there is less air above you pushing down. Even at a fixed location, the air pressure varies just a bit from day to day. Low pressure often indicates bad weather on the horizon, while high pressure portends a sunny day. Thus the barometer became an important instrument for short term weather forecasting. It's better than anything they had before, which was nothing.
Ok, let's return to your swimming pool. You can suck water 3 feet up the tube, but how far down can you blow? Maybe your lungs are stronger blowing out than sucking in. Sorry, they aren't. Not surprising, since it's the same muscle (diaphragm) after all. You can push air down the straw about 3 feet below the level of the water, and that's it. There's no blowing bubbles through a 6 foot straw.
Perhaps, in one of your heroic day dreams, you find someone who is trapped below water. His leg is caught in something and he can't come up for air. Quick thinking, you come to the rescue with a hose. That works, as long as his head is only a foot under water. If he is pinned several feet below the surface, the hose is useless. His diaphragm isn't strong enough to pull air down to that depth. You better think of something else.
Every time I've had a tooth out, my dentist gives me the same list of instructions, and one of them is, “Don't drink through a straw for a few days.” The low pressure in the mouth, created by sucking on a straw, can break open the fresh wound, and/or cause it to bleed. Ok, but I like drinking through a straw. I never use open glasses; I always use an M-cup from McDonalds. If I knock it over, (I am sometimes a klutz), nothing spills. And I can't inadvertently drop crumbs into my cup. So, after an extraction, I go straight home and drink out of my M-cup as usual. But here's the thing, I understand physics. Plus I'm cocky. And I'm stubborn. It's the way I like to drink. I'm drinking water, not a thick vanilla shake, so I can calculate the pressure difference from my sucking. It is proportional to the height that I must lift the water through the straw. If I fill the cup all the way to the top, I'm only sucking up a couple of inches. Remember that 30 feet of water is approximately one atmosphere of pressure. Therefore, 2 inches is 1/180 atmospheres of pressure, or 0.083 psi. Nothing bad is going to happen from 0.083 psi. When my cup drinks down a bit, I fill it back up again, so the height differential is never more then 4 inches, never more than 0.2 psi. There, science will triumph. Disclaimer: if the straw has even a microscopic crack above the waterline, it takes considerably more suction to drink the water, so make sure your straw is an unbroken tube.
The barometer has inspired yet another unit of pressure, which is based on the element mercury. One atmosphere raises a column of mercury 30 inches high, or 762 millimeters high. Therefore, 1 atm = 15 psi = 30 inches of mercury = 762 millimeters of mercury. Half an atmosphere, as you would find 5,500 meters up the side of a mountain, supports 381 millimeters of mercury, and so on. Millimeters of mercury, or mm, is a new unit of pressure that is typically used in medical circles.
If the doctor says your blood pressure is 135 over 85, what does that mean? The unspoken units are millimeters of mercury. With each beat, the heart forces blood through the arteries. The blood is under pressure as the ventricle contracts, the same pressure that would raise a column of mercury 135 millimeters high. The doctor could say 0.177 atm, or 2.65 psi, and it would mean the same thing. After the beat, the heart relaxes, but still the blood is under pressure, holding the arteries taut. This is the lesser number of your blood pressure. Your arteries are always under a baseline pressure of 85 mm. Prolonged exposure to high blood pressure can cause an artery to burst, leading to a stroke or aneurism.
In 18th century Japan, a Samurai would sometimes test the quality of his sword by slicing off the head of a random passerby. Assuming the victim remains upright, at least for a short time, how high does the blood spurt from his carotid arteries? Science provides an answer, or at least an upper limit.
Blood pressure is measured from the heart, which is why those wrist machines tell you to “Raise your wrist to the heart level.” Our calculations must account for the distance between the heart and the top of the neck. The heart squeezes with a force of 135 millimeters of mercury, but it is squeezing blood, not mercury. Mercury is 13.5 times as dense as blood, so multiply by 13.5 and get 1,822 millimeters, or 1.8 meters, or 6 feet. Suppose the carotid artery continued, at the same diameter, out of the neck and up into the sky. With each beat, blood climbs this artery 1.5 meters, or 5 feet, beyond the neck. In between beats, as the heart relaxes, the blood drops to a level of 1 meter, or 3 feet, above the neck. blood pulses up and down with each beat until the heart stops. Of course the artery does not rise up into the sky like a macabre barometer, thus the blood is not contained in a nice neat column. It spreads out and is dispersed in the air. It will not rise as far as an extended artery would dictate. A reasonable guess might be 1 meter, or 3 feet, above the neck. Levels will vary depending on the victim's blood pressure at time of death.
Star Trek used millimeters of mercury to describe atmospheric pressure in one episode, The Galileo 7. As you recall, the shuttle craft landed on an uncharted planet, with Spock in command. McCoy began by taking a reading on the atmosphere with his tricorder. He reports oxygen at 70 mm and nitrogen at 140 mm. “Breathable, if you're not running in competition.” he declares. However, the total pressure is 210 mm, not 760 mm. It's just over ¼ of our atmosphere, the pressure at 30,000 feet, where airplanes fly. It is not "breathable", not at all. The crew could not step outside without supplementary oxygen, and yet they do. Oops.
As you gain altitude, atmospheric pressure drops exponentially. Thus our atmosphere, or any atmosphere, has a half life of sorts. On earth, pressure drops by half every 18,000 feet, or 5,500 meters. Climb a mountain 18,000 feet high, and pressure is half what it was at sea level. The air is half as dense. At 36,000 feet, twice 18,000 feet, where airplanes fly, the air is a fourth as thick, a fourth as dense, as it is at sea level. At 54,000 feet the pressure is one eighth atm, one eighth the pressure at sea level. Even this is too thick for supersonic flight, as the plane would overheat, so the Concorde flew at 60,000 feet. 60/18 = 3.33, 23.33 = 10, thus a tenth the pressure at sea level. Rising further into the sky, each 18,000 feet cuts the pressure in half, until it is merely wisps of air molecules marking the border between air and space.
|
If you're familiar with differential equations, then atmospheric half life is easy to prove. At any altitude x, let y be the amount of air pressing down from above. Hence y is a decreasing function of x. The weight of the air above is the pressure, and if we make a few simplifying assumptions, like constant temperature, then pressure is proportional to density. Density in turn is the change in y with respect to x. Thus ∂y∂x is proportional to y, and that produces an exponential curve. |
Let's come back down to earth and ascend more slowly. Climb a mountain, by car and then on foot, to an altitude of 13,000 feet, or 4,000 meters, as I did when I climbed the mountains of Utah with my grandfather. My ears gently popped as we climbed, easily addressed by swallowing. The thin air, 65% of normal, didn't bother me, but my grandfather was panting just a bit. There is less air per cubic meter, and less oxygen per cubic meter, and less oxygen for the blood. Experienced climbers, or people who live at high elevations, can breathe unassisted, without supplementary oxygen, up to 18,000 feet, 50% pressure. Everyone needs help beyond that point.
Turning to aviation, planes that fly above 12,000 feet have closed cockpits with oxygen on board. A blackout, while flying in a plane, doesn't end well. Planes that fly above 24,000 feet, 40% pressure, are sealed and pressurized. A human can live at low pressure with supplementary oxygen if the change is gradual, but a plane can reach 24,000 feet in a hurry. Without time to adjust, the pilot's eardrums could burst. The same problem occurs in reverse when the plane descends, and returns to standard pressure in just a few minutes. The pilot's ears could implode. Thus a commercial airplane is held at 70% atm, the pressure found at 8,000 feet. This is a compromise between passenger comfort and the strength and weight of the fuselage that contains the pressurized air.
At a cruising altitude of 36,000 feet, outside pressure is 25%, and pressure in the cabin is 70%. If a nutjob opens the window,the pressure drops by 6.75 psi in under a minute. Eardrums burst, and in some cases, blood vessels burst in the nose and mouth. This is survivable, but very painful, and some folks may have hearing loss for the rest of their lives. That is problem number 1.
Whoever opened the window, and the two passengers sitting next to him, probably got sucked out of the plane. More accurately, they were blown out by the air inside as it forced its way through the opening, like air out of the tail of an inflated balloon. Bye Felicia. When planes have ruptured due to mechanical failure, entire rows of seats, along with their belted passengers, have flown out into the sky.
After the passengers adjust to the low pressure, they realize there isn't enough oxygen to survive. That's what the oxygen masks are for, as they drop down from the ceiling. Remember to put yours on first, then assist others. If you can't get to a mask in time, you could pass out and die.
The temperature is well below zero at this altitude. Obviously the pilot is going to descend as quickly as he can, but for now, you're in a deep freeze, and you probably don't have a coat or a hat. If you're anywhere near the window, you'll feel a wind chill like you've never felt before. Frostbite and hypothermia are almost guaranteed.
Any opening in the fuselage disrupts the aerodynamics of the plane, especially at cruising speeds. The high speed wind could damage the plane, or at the very least, blow personal items about the cabin and injure the passengers. Naturally the pilot decreases air speed as quickly as possible, but those first few minutes are critical.
And that, my friends, is why you can't open the windows on an airplane.
The atmosphere on Venus is almost all carbon dioxide, and since carbon dioxide is heavier than air, the half life is less. As you gain altitude, pressure is cut in half every 11,500 feet, or 3,500 meters. If you lived on Venus, and there was an Empire State building, and you rode the elevator up to the top floor, say 350 meters, pressure would change by 7.5%. That doesn't sound like much, but remember, pressure at the ground floor is 90 atmospheres. A small change makes a big difference! Pressure drops by 6.75 atm in just a couple minutes, and that will rupture your eardrums for sure, and probably give you the bends. Even a 2 story building is impractical. climbing a flight of stairs changes the pressure by half an atmosphere, more than the aforementioned plane bursting open in flight. Heck, just sitting up in bed will pop your ears. I suppose the entire building could be held at 1 atm inside. We're already keeping the heat and carbon dioxide out, and maintaining an oxygen atmosphere within, so why not maintain one atmosphere of pressure as well? That works, but the building has to have mighty strong walls to stand against the pressure outside. It probably has to be a dome.